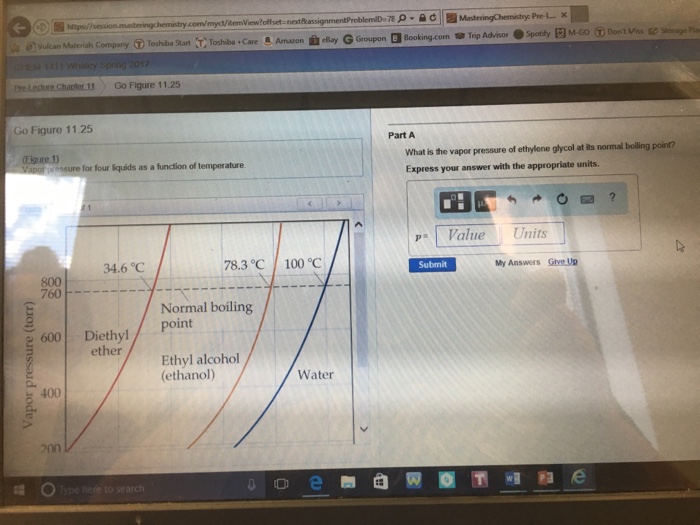
Vapor Pressure of Ethylene Glycol at Its Normal Boiling Point
To form a hydrogen bond what must the nonhydrogen atom NO or F involved in the bond posses. See the answer See the answer See the answer done loading.
Solved Vapor Pressure Of Liquids As Function Of Temperature Chegg Com
AsH3 has a higher boiling point due to its larger molecular weight.
. This is illustrated in the vapor pressure chart see right that shows graphs of the vapor pressures versus temperatures for a variety of liquids. What is the vapor. Read Online Determination Of Boiling Point Of Ethylene Glycol associated with the analy tical chemist finds its greatest opportunity for application in the problems of cognition and.
We are trying to calculate the vapor pressure of. The osmotic pressure of blood is 765 atm at 37 C. 6207 NTP 1992 Water Solubility.
Find the vapor pressure formula here. The vapour pressure can be brought down by refrigeration and this is the way in which ethylene is usually transported by. Of Ethylene Glycol Determination Of Boiling Point Of Ethylene Glycol When people should go to the book stores search instigation by shop shelf by shelf it is in point of fact problematic.
What is the vapor pressure of ethylene glycol at its normal boiling point. In Example PageIndex1 we calculated that the vapor pressure of a 302 aqueous solution of ethylene glycol at 100C is 851 mmHg less than the vapor pressure of. 1973 deg C.
What is the vapor pressure of ethylene glycol at its normal boiling point. This problem has been solved. Other uses Dehydrating agent.
What is the vapor pressure of ethylene glycol at its normal boiling point a 260 from CHEM 1412 at Southern Methodist University. What is the vapor pressure of an aqueous solution of the nonelectrolyte ethylene glycol in water at 25C if its normal boiling point is 1018C. What is the vapor pressure of ethylene glycol at its normal boiling point.
By comparison ethyl alcohol CH 3 CH 2 OH. At that temperature its vapour pressure is approximately 50 bar. What is the vapor pressure of ethylene glycol at its normal boiling point.
Greater than or equal to 100 mgmL at 635F NTP 1992. At the normal boiling point of a liquid. The specific heat of ethylene glycol based water solutions are less than the specific.
Up to 256 cash back Ethylene glycol HOCH 2 CH 2 OH the major substance in antifreeze has a normal boiling point of 198 C. A solution of ethylene glycol in water such as in an antifreeze solution has a boiling point 100C and a freezing point 0C. 126 kgm 3 27315K.
Freezing point 100 ethylene glycol at atmospheric pressure is -128 o C 9 o F 1 Btulb m o o C Note. Temperature and Vapor or Saturation Pressure for some common Fluids. Lower vapor pressure are called heavy components.
Ethylene Glycol CAS RN. Find the equations of the tangent and the normal to the given curve at the indicated point for y x3 - 2x7 at 1 6. Ethylene glycol is used in the natural gas industry to remove water vapor from natural gas before further processing in much the same manner as triethylene.
Learn how to calculate vapor pressure of ethanol using the Clausius-Clapeyron equation for pure liquids. 3877F at 760 mmHg NTP 1992 Molecular Weight. Since the normal boiling point is the temperature at which the vapor pressure equals atmospheric pressure at sea level we know one vapor pressure-temperature value T1 801 C 3533 K.
Previous which level of party organization is most responsible for ing the partys nominee win the. At atmospheric pressure saturation temperature of. What is the vapor pressure of ethylene glycol at its normal boiling point.
The Kb for water is 052Cm and.

Ethylene Density And Specific Weight Vs Temperature And Pressure

Triethylene Glycol Dimethyl Ether Cas 112 49 2 808249
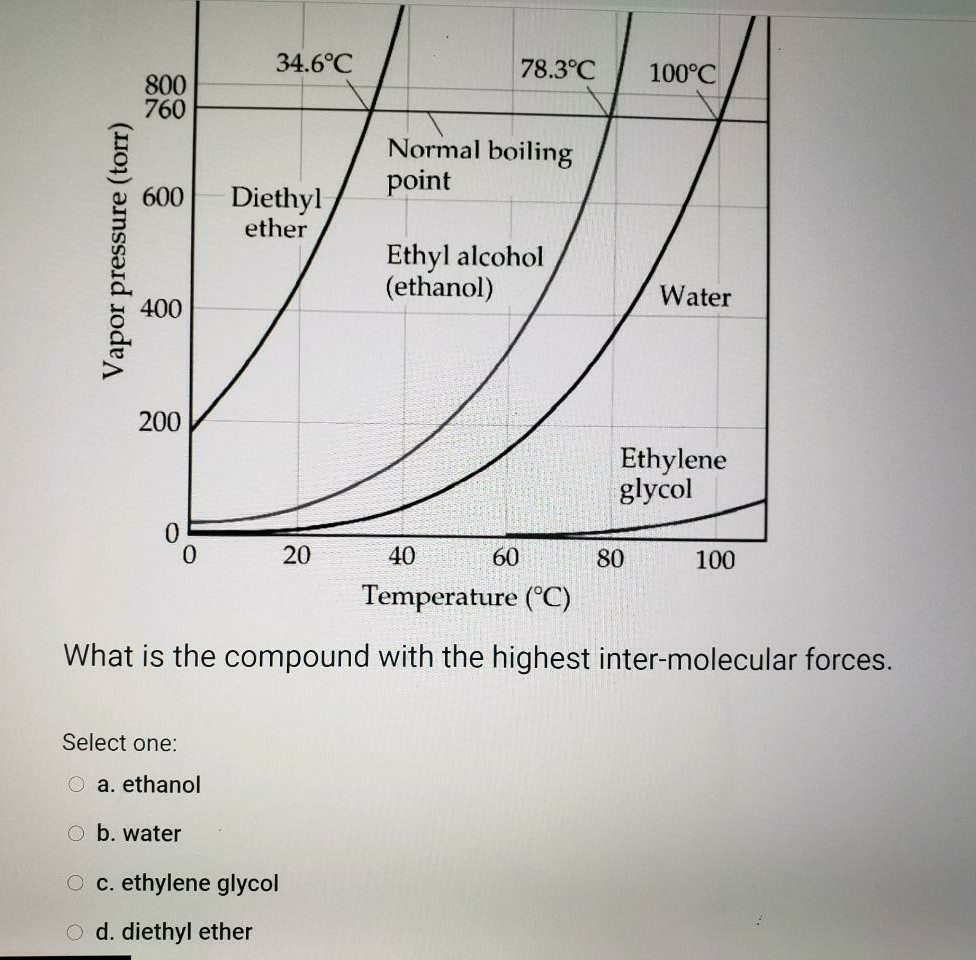
Solved 34 6 C 78 3 C 100 C 800 760 Normal Boiling Point 600 Chegg Com
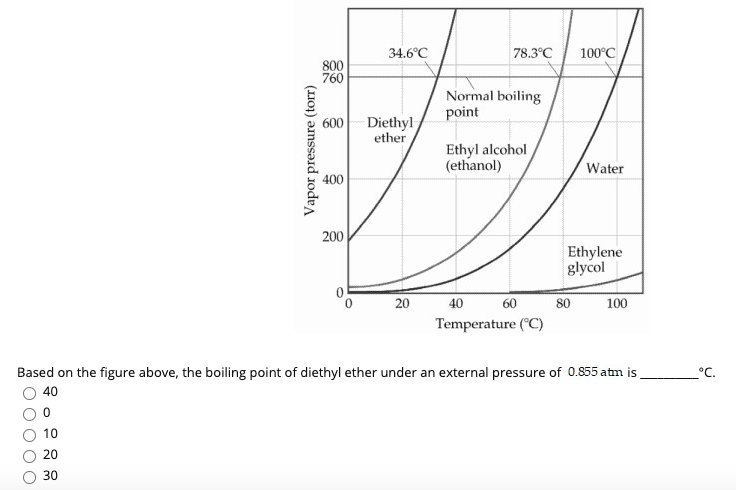
Solved 34 6 C 78 3 C 100 C 800 760 Normal Boiling Point 600 Chegg Com
Comments
Post a Comment